AGRiKA™ technology and its use in agriculture industry
Problem of soil salinization
The beginning of 21st century is marked by global scarcity of water resources, environmental pollution and increased salinization of soil and water. Increasing human population and reduction in land available for cultivation are two threats for agricultural sustainability. Various environmental stresses viz. high winds, extreme temperatures, soil salinity, drought and flood have affected the production and cultivation of agricultural crops, among these soil salinity is one of the most devastating environmental stresses, which causes major reductions in cultivated land area, crop productivity and quality. The area of land in the United States of America affected by soil salinity and drought is increasing day by day.


Problem of soil acidification
Soil acidity is a major environmental and economic concern. Soil acidification occurs because the concentration of hydrogen ions in the soil increases. If untreated, acidity will become a problem in the subsurface soils, which are more difficult and expensive to ameliorate. Subsurface acidity is already a major problem for large areas of California, Nevada, Montana, Texas. Acidic soils cause significant losses in production and where the choice of crops is restricted to acid tolerant species and varieties, profitable market opportunities may be reduced. In pastures grown on acidic soils, production will be reduced and some legume species may fail to persist. Soil acidification occurs naturally very slowly as soil is weathered, but this process is accelerated by productive agriculture.
Over-Fertilization of Soils
Over-fertilization of soils used for agricultural and horticultural purposes is a growing environmental concern. The problem results in high concentrations of soluble salts in the soils. These salts damage roots by slowing the net flow of water into the roots and indirectly by predisposing the plants to certain root diseases and damping-off. Nutrient levels that are above optimum do not improve plant growth. In addition, excessive nutrients can cause adverse effects on plant growth, increase the potential for environmental contamination due to leaching, and represents a waste of resources.


Air pollution affected Soils sustainability
Rainwater is naturally acidic as a result of carbon dioxide (CO2) gas (which is naturally present in the atmosphere), dissolved in water. Rainwater can become more acidic when man-made pollutants also become dissolved. This acidity is mainly derived from the washout of oxides of sulphur (SO2) and nitrogen (NO2). Although these compounds exist naturally in the environment, by-products of burning fuels such as coal and oil now form the largest source of man-made acidity in the atmosphere. Once in the atmosphere, these oxides can mix with other chemicals to form more harmful pollutants that either settle as ‘dry deposition’ or are washed out by rain and fall as ‘wet deposition’. The most significant man made acids in precipitation are sulphuric (H2SO4) and nitric (HNO3) acid. Most of the SO2 and NO2 produced in the United States of America come from power stations and large industrial units, but cars and heavy vehicles are also important sources of the oxides of nitrogen.
AGRiKA concept
AGRiKA concept is a way to raise the yield by removing pesticides and fertilizers from sick soil of an agricultural field by increasing profit and efficiency of farmer’s investment.


Salinity problems
Irrigation is an ancient and important agricultural practice. Crop yields are higher under irrigation and less dependent on the effects of weather.
Irrigation inevitably leads to the salinization of soils and waters. In the United States yield reductions due to salinity occur on an estimated 30% of all irrigated land.
Application of irrigation water results in the addition of soluble salts such as sodium, calcium, magnesium, potassium, sulphate, and chloride dissolved from geologic materials with which the waters have been in contact. Evaporation and transpiration (plant uptake) of irrigation water eventually cause excessive amounts of salts to accumulate in soils. Excessive soil salinity reduces yields by lowering plant stand and growth rate. Also, excess sodium under conditions of low salinity and especially high pH can promote slaking of aggregates, swelling and dispersion of soil clays, degrading soil structure and impeding water and root penetration. Some trace constituents, such as boron, are directly toxic to plants.
Salinity problems reduce productivity on both irrigated and non-irrigated agricultural lands in the United States and throughout the world.
Soil acidification
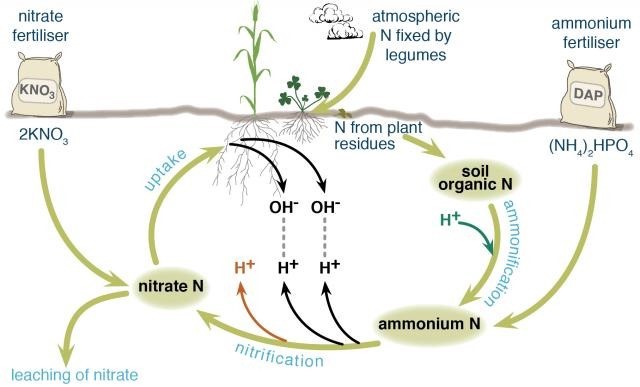
Ammonium-based fertilisers are major contributors to soil acidification.
Nitrogen in agricultural systems may be fixed from the atmosphere by legumes, decomposed from soil organic matter (the dead remains of plants and animals) by soil organisms, or added in various types of fertilisers. Different nitrogen fertilizers follow slightly different chemical pathways as they break down in the soil and contribute different amounts of hydrogen ions (acid) to the soil.
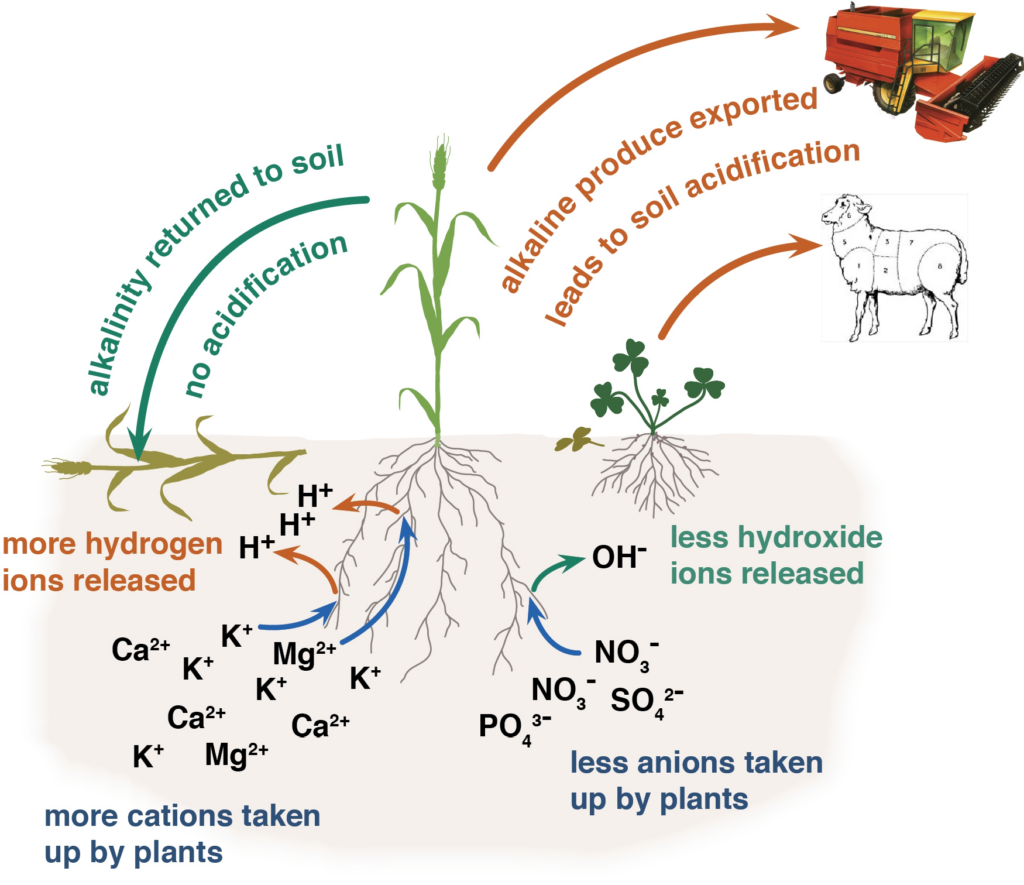
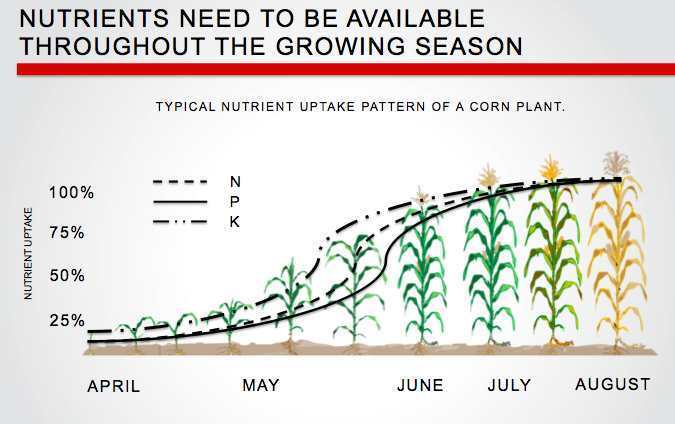
Fertiliser nitrogen that enters and leaves the system in the same form does not contribute to soil acidification, for example, potassium nitrate. Nitrogen that stays in the system does not contribute to soil acidification, for example, nitrogen added to the soil as fertiliser is taken up by a plant and then returned to the soil when the plant dies and decomposes and is taken up by another plant and so on (Figure 1).
Plant roots take up nutrients as either cations, which are positively charged (such as ammonium, potassium, calcium or magnesium) or as anions, which are negatively charged (such as, nitrate, phosphate or sulphate). When a cation is absorbed by a plant, a positively charged hydrogen ion is be excreted into the soil to maintain electrical balance. When an anion is absorbed, a negatively charged hydroxide ion is excreted into the soil.
Plants absorb more cations than anions, which means that most plant material is slightly alkaline. In a natural system, when plants die they are decomposed and returned to the soil, balancing the acidity caused by the hydrogen ions.
In agriculture, if plant material is removed by grazing or harvest or relocated by the concentration of dung into stock camps, rather than being returned to the soil, there is a net export of alkalinity and residual hydrogen ions remain in the soil contributing to soil acidity (Figure 2). Over time, as this process is repeated, the soil becomes acidic. A translocation of alkalinity can occur in windrows with the soil off the windrow becoming more acidic.
Plant growth and most soil processes, including nutrient availability and microbial activity, are favoured by a soil pH range of 5.5 – 8. Acid soil, particularly in the subsurface, will also restrict root access to water and nutrients.

Aluminium toxicity
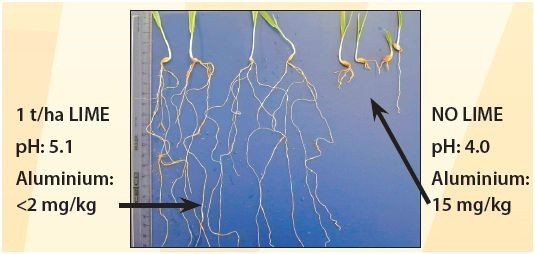
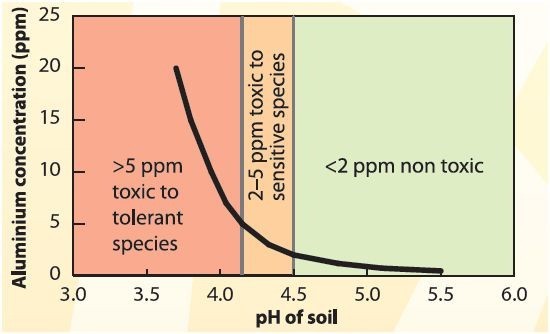
When soil pH drops, aluminium becomes soluble. A small drop in pH can result in a large increase in soluble aluminium (figure 1). In this form, aluminium retards root growth, restricting access to water and nutrients (figure 2).
Poor crop and pasture growth, yield reduction and smaller grain size occur as a result of inadequate water and nutrition. The effects of aluminium toxicity on crops are usually most noticeable in seasons with a dry finish, such as drought in California, Nevada and other states, as plants have restricted access to stored subsoil water for grain filling.
Nutrient availability
In very acid soils, all the major plant nutrients (nitrogen, phosphorus, potassium, sulphur, calcium, manganese and also the trace element molybdenum) may be unavailable, or only available in insufficient quantities. Plants can show deficiency symptoms despite adequate fertiliser application.
Microbial activity
Low pH in topsoils may affect microbial activity, most notably decreasing legume nodulation. The resulting nitrogen deficiency may be indicated by reddening of stems and petioles on pasture legumes, or yellowing and death of oldest leaves on grain legumes. Rhizobia bacteria are greatly reduced in acid soils. Some pasture legumes may fail to persist due to the inability of reduced Rhizobia populations to successfully nodulate roots and form a functioning symbiosis.
Over-Fertilization
There are several reasons why soil might be over-fertilized. Human activity is often the cause of excessive nutrient levels, including over-fertilization, or the overuse of compost, manure or other organic materials. It has become increasingly common for soil tests processed by various US agriculture laboratories from gardens and small farms to have excessive nutrient levels.
Effects of Over-Fertilization: Optimum nutrient levels listed on soil test results represent the range at which plant growth is maximized. Nutrient levels that are above optimum do not improve plant growth. In addition, excessive nutrients can cause adverse effects on plant growth, increase the potential for environmental contamination due to leaching, and represents a waste of resources.
In particular, above optimum nitrogen and phosphorus levels can lead to excessive plant and algal growth in waterways that can degrade drinking water, fisheries, and recreational areas. High potassium can lead to an imbalance of base saturation levels as well as high soluble salts. High calcium and magnesium levels are commonly associated with pH values above 7.0.
In addition, high organic matter levels can cause poor drainage. Areas where lawns or turf are being grown should have organic matter levels less than 5%. In general, organic matter levels greater than 8% in outdoor growing environments are unnecessary and can cause some of the issues listed above.
Remediation of Over-Fertilization: Application of AGRiKA™ technology can correct over-fertilization with immediate effect. The huge accumulation of old solidified fertilizer residues, which are in the over-fertilized soils will became fossilized, breached down to the constituent parts, which then absorbed by the roots of the plants as highly nutrient components.
Before

After

CALIFORNIA DROUGHT PROBLEM
California has grappled with the drought for the last five years. Most of Southern California and several central areas of the state remain in extreme and exceptional drought. More than 26 million people are in drought-stricken areas.
Parched conditions fueled numerous, deadly wildfires across the state. More than 102 million drought-stricken trees in the state have dried up and died since 2010, the USDA estimated. Many homes have run out of running water because of dried up wells. It also has devastated farms, forcing layoffs of thousands of farm workers because of reduced water allocations. In 2014, California Gov. Jerry Brown declared a drought emergency and warned that the state was facing “perhaps the worst drought that California has ever seen since records (began) about 100 years ago.“ The next year, he imposed the first ever mandatory water restrictions on residents, businesses and farms. He also ordered cities and towns to reduce usage by 25% in 2015.
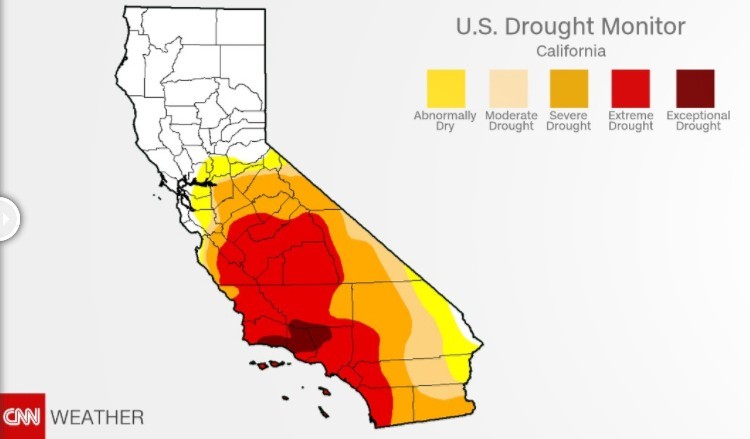
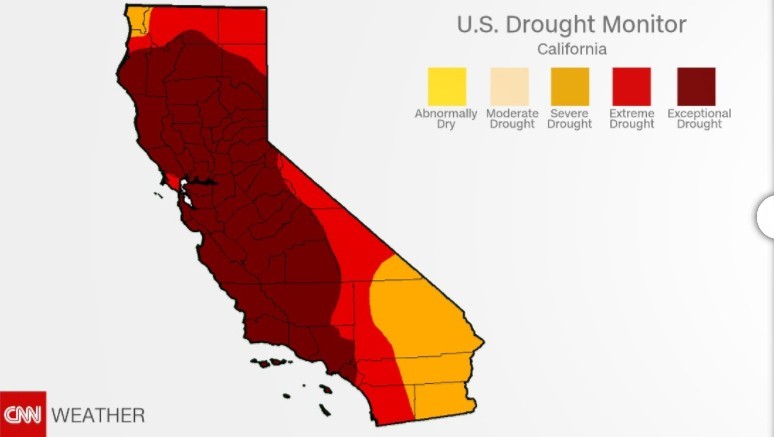
State of California: July 2014 State of California: January 2017
After three consecutive years of drought, California farmers can easily see the impact on agriculture. Now more than ever, growers need to combat numerous root-level challenges that come with severely dry conditions. While salinity is usually a top-of-mind issue for crop health, drought conditions can quickly accelerate salinity problems in soils. High salinity levels can cause lower productivity and performance – with a real impact on profits.
Drought drives high salinity
Soil salinity levels increase during extended drought periods because less water is available to leach salts (salt already present in soil), which can lead to an abundance of concentrated salt. When soil salinity levels are high, water in the roots is pulled out and back into the soil, depriving the plant of any available moisture and causing potential loss in growth and productivity.
Drought drives high salinity
Soil salinity levels increase during extended drought periods because less water is available to leach salts (salt already present in soil), which can lead to an abundance of concentrated salt. When soil salinity levels are high, water in the roots is pulled out and back into the soil, depriving the plant of any available moisture and causing potential loss in growth and productivity.
MEGADROUGHTS A THREAT TO CIVILIZATION – CALIFORNIA’S 100-YEAR DROUGHT
Megadroughts are extreme dry spells that can last for a decade or longer. They have parched the West, including present-day California, long before Europeans settled the region in the 1800s.
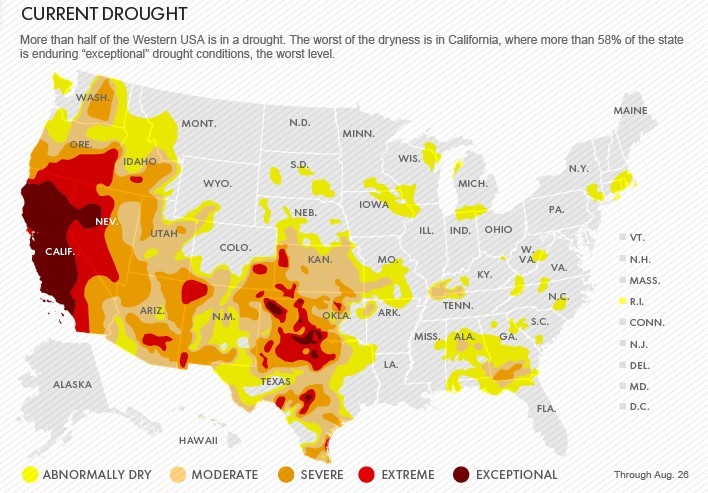
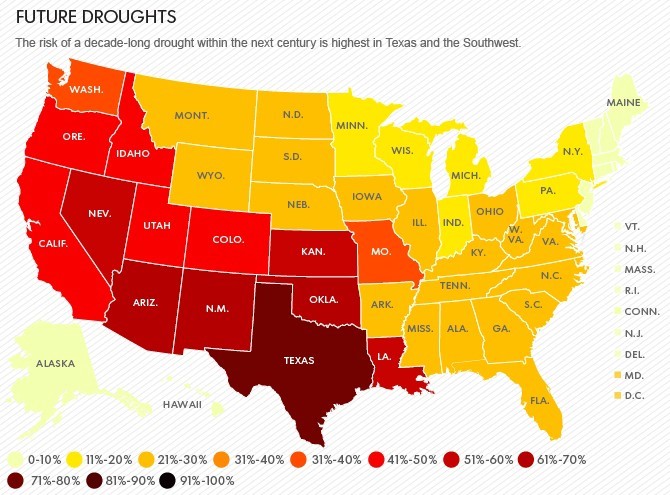
The coming megadroughts will have profound effects on water resources and agricultural productivity. Water rights in the West were carved up during the 1920s, one of the wettest periods in the past 500 years. And the West’s population explosion between 1980 through 2000 also coincided with wetter than normal decades. Now, with nearly every drop of surface water legally claimed, cities and famers make up any deficit by tapping non-renewable underground aquifers, which are already straining to meet current demands.





